Membrane Technology for a Sustainable World
immc | Louvain-la-Neuve


Following the principle of membrane deployment for a more sustainable society, our lab has joined the global effort to address climate change. So far, the work has been focused on carbon capture and its valorization in the form of bicarbonate salts and/or organic molecules. First, carbon capture is performed by absorbing CO2 in a gas-liquid membrane contactor set-up. Greener solvents are of interest for the absorption, although they are often kinetically limited. Current work addresses the enhancement of the absorption by promoting the solvents with amino acids or enzymes (either dissolved in the solvent or immobilized on the membrane surface). Envisaging a more realistic study of the process performance, the effect of SO2 and NOX in the effluent gas to be treated by the membrane contactor is also considered. After the absorption step, the bicarbonate salts must be separated from the homogenous catalysts, i.e. dissolved enzymes or amino acids, either by ultrafiltration or nanofiltration processes. This allows the reutilization of the catalysts. In the case of enzyme immobilized on the membrane surface, this step can be avoided. The final product is obtained after concentration and crystallization of the aqueous bicarbonate solution using membrane crystallization (OMD, DCMD or VMD).

Researcher: Mar Garcia Alvarez
Upgrading CO2 into a chemical compound is a way of moving towards carbon circularity. Formate is the first reduced form of CO2 and is the basis for the synthesis of many other molecules. Converting CO2 using enzymes enables higher conversion rates to be achieved at a lower environmental cost. A method for converting CO2 into formate is inspired by a known biochemical route consisting of a redox reaction between CO2 and NADH in the presence of formate dehydrogenases (FDH) as catalysts. NADH has a high economic cost, hence, it is necessary to regenerate it during the process. The conversion of CO2 into formate is catalyzed by FDHs that are immobilized on the membrane to increase their stability. The development of a process converting CO2 into formate while regenerating NADH would enable to have a continuous production of formate from CO2, the environmental and economical cost having to be as low as possible.
Researcher: Ysaline Toussaint
Pressure-driven membrane processes, such as nanofiltration (NF) and reverse osmosis (RO) hold great promise for heavy metal removal due to their ease of use, small footprint and high efficiency. Nanofiltration is a membrane-based process used to remove metals from wastewater and colloidal materials, operating at low transmembrane pressures. Recently, RO membranes operating at ultra-low pressures, similar to nanofiltration have been developed. In this research line, our work focuses on the elimination of micro-pollutants from household and industrial effluents to WHO standards and the permeate can be reused. Furthermore, a hybrid process of RO or NF coupled with membrane crystallization (MCr) is being investigated to recover useful compounds from the retentate. Finally, anti-biofouling nanofiltration membrane separator in microbial fuel cells (MFCs) is also being explored to treat textile wastewater.

Electro-driven membrane processes for high-salinity organic wastewater treatment
A critical requirement for the sustainable management of high-salinity organic wastewater is the precise fractionation of organic compounds and inorganic salts (NaCl) as recoverable resources. Conventional membrane processes, including electrodialysis, nanofiltration, and ultrafiltration, are often constrained by severe membrane fouling, which undermines organic/salt separation efficacy. To address this limitation, a novel electro-driven membrane process is proposed that integrates the advantages of electrodialysis and pressure-driven separation. In this process, conventional anion exchange membranes are replaced with nanoporous membranes serving as anion-conducting membranes, enabling fast desalination of organic streams while effectively mitigating fouling-induced performance loss. Ongoing research focuses on optimizing membrane design to further enhance ion transfer rate, organic/salt selectivity, and long-term operational stability.
Researchers: Zijian Yu
Design and fabrication of sustainable antifouling membranes for efficient removal of micro- and nanoplastics
The study focuses on developing eco-friendly membranes using biodegradable or renewable materials to address environmental concerns associated with conventional polymeric membranes. By precisely optimizing membrane structure and surface chemistry, the work seeks to enhance antifouling performance and maintain high filtration efficiency. The surface morphology, chemical composition, and hydrophilicity of the fabricated membranes will be systematically characterized, and their separation and fouling behaviors will be evaluated. This research is expected to provide a sustainable strategy for mitigating microplastic pollution and contribute to the development of environmentally friendly water purification technologies.
Researchers: Shuangling Xie
Membrane process for the removal and degradation of PFAS “forever chemicals” from treated wastewater
This research line focuses on developing advanced membrane-based processes for the removal and degradation of persistent organic pollutants, particularly per- and polyfluoroalkyl substances (PFAS), also known as “forever chemicals.” The work explores the integration of two strategies: nanofiltration (NF) with (bio) catalytic systems, including enzymatic and photocatalytic approaches, to enhance both pollutant separation and chemical breakdown. Special attention is given to real wastewater effluents, aiming for high-quality treated water. Our goal is to design scalable, sustainable, and energy-efficient membranes that destroy PFAS safely—ideally achieving complete mineralization—while preventing the formation of toxic byproducts, supporting water reuse and environmental protection.

Researchers: Estefanía Aguirre Álvarez
Design and fabrication of novel electro-nanofiltration membranes for sustainable management of hypersaline organic wastewater
The sustainable method to treat hypersaline organic wastewater remains a significant challenge in industrial wastewater management. Conventional electrodialysis and nanofiltration processes plagued by membrane fouling, which hampers their efficiency in separating organics and salts. In response to this challenge, this study focuses on the design and fabricate novel electro-nanofiltration membranes with remarkable organics/salt selectivity and excellent antifouling performance, for the sustainable treatment of hypersaline organic wastewater. Furthermore, the study explores the optimization of preparation conditions to precisely tailor membrane surface properties (e.g., surface charge and pore size) and evaluate their performance in different applications.
Researchers: Weishuang Yuan
Lithium Extraction from Salt Lake Brines: A Membrane Separation Approach
The lithium used in lithium-ion batteries primarily comes from mining and recycling. Currently, global lithium supply is dominated by mining, which accounts for approximately 95%–98%. Among these sources, lithium extraction from salt lake brines represents about 60%–70%. Lithium-rich brines or salt lakes contribute the majority of global lithium carbonate production and all lithium chloride production. Extraction from these salt lakes is considered the most convenient and cost-effective method. However, salt lake brines also contain many other impurity ions, particularly magnesium. Therefore, efficiently extracting lithium from brines with high magnesium concentrations has become a major challenge. This study focuses on lithium–magnesium separation based on electrodialysis (ED) technology, employing an ion-“distillation” strategy to achieve selective ion separation. By designing ion-exchange membranes with enhanced selectivity and reduced energy consumption, this research aims to enable green and efficient lithium extraction.
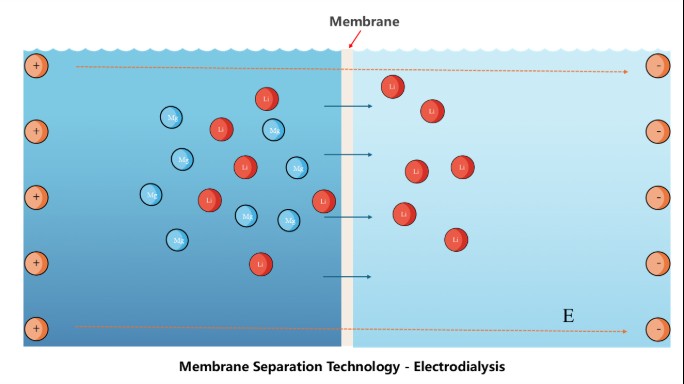
Researchers: Qian Xu
Different applications require distinct membrane properties. In our laboratory, some processes utilize commercial membranes but some lines of research investigate the preparation of novel membranes with the desirable characteristics.
Some examples are given below.
Biocatalytic composite membranes for gas-liquid membrane contactors
Gas-liquid membrane contactor is one of the promising membrane separation technologies for carbon dioxide capture. When greener solvents are used, the carbon capture efficiency decreases significantly due to the slower kinetics of these greener solvents. Combining biocatalysts or enzymes with these green solvents could boost the carbon capture efficiency while allowing for conversion of capture carbon dioxide into useful products. In this research line, we are developing immobilization platform for biocatalysts or enzymes on the membrane surface.
Researcher: Ysaline Toussaint
Composite and mixed matrix membranes for pervaporation/reactive pervaporation and water purification
Composite and mixed matrix membranes are advanced material designed for pervaporation, reactive pervaporation and water purification processes that typically require dense selective layers. By combining polymers with fillers such as MOFs and zeolites these membranes offer enhanced selectivity, permeability, and stability. In pervaporation systems, they enable efficient separation of complex or azeotropic mixtures, while in water purification, they improve hydrophilicity, antifouling behavior, and contaminant removal. Overall, composite and MMMs provide a versatile and sustainable platform that bridges the gap between polymeric and inorganic membrane technologies.
Researcher: Akshara Iyer
Porous membranes for membrane crystallization
In our lab, we focus on developing sustainable porous membranes for membrane crystallization (MC) — an innovative and environmentally friendly process that integrates separation and crystallization to produce high-purity crystals efficiently. Our goal is to design eco-friendly, energy-efficient membrane systems that contribute to green process engineering and sustainable manufacturing. We synthesize our membranes using green solvents and biopolymers and environmentally benign materials, reducing reliance on hazardous chemicals during fabrication. This green synthesis approach minimizes environmental impact, enhances membrane performance, and promotes safer, cleaner production methods. By precisely controlling mass transfer and supersaturation through tailored porous structures, our research enables uniform crystal growth, improved purity, and reduced energy consumption. The sustainable membranes developed in our lab have potential applications in pharmaceutical manufacturing, water desalination, and chemical processing, supporting the global transition toward cleaner and more sustainable technologies.
Researcher: Syeda Laraib
Sustainable and PFAS-free proton exchange membrane for green H2 production
A sustainable, PFAS-free proton exchange membrane (PEM) is being developed to replace conventional materials like Nafion, which are costly and environmentally persistent. Serving as a key component in electrochemical systems, the PEM facilitates proton transport, maintains pH balance, and prevents gas crossover. Bio-based and hydrocarbon polymers show strong potential as sustainable alternatives, offering high proton conductivity and good chemical and mechanical stability. Such membranes can enable cleaner and more efficient technologies for energy conversion and wastewater treatment.
Researchers: Andreas

Reactive pervaporation
The research is a hybrid process of combined transesterification reaction and pervaporation in one setup, which contains three steps:
- development of methanol (MeOH)-selective pervaporative membranes,
- utilization of the developed MeOH-selective membranes in reactive pervaporation setup, and
- development of catalytic and MeOH-selective membranes.

Researcher: Akshara Iyer
Membrane distillation-crystallization takes advantage of the membrane that can allow a non-dispersive contact between two streams, which leads to the progressive distillation of the feed stream and eventually provokes crystallization of the salt. Different configurations are investigated: osmotic, direct contact and vacuum membrane distillation-crystallization. Recently, the use of a focused beam reflectance measurement (FBRM) probe allows us to follow the crystallization process on the membrane surface.

Researcher : Syeda Laraib
As sustainability lies at the core of our research, one of our key focus areas is the quantification and reduction of environmental impacts across the life cycle of materials, products, and technologies. Life Cycle Assessment (LCA) is a powerful tool to ensure that efforts to reduce greenhouse gas emissions do not inadvertently cause other environmental burdens, such as land degradation or acidification.
Within this context, our research explores the integration of artificial intelligence (AI) and machine learning (ML) into LCA to support the development of sustainable Materials-by-Design for renewable energy systems. Traditional LCA approaches can be time-consuming and uncertain, especially for emerging technologies with limited data. By leveraging AI-based prediction models, we aim to improve the accuracy and efficiency of environmental impact assessments for novel material systems used in energy conversion and storage.

The research focuses on developing an integrated framework capable of predicting life cycle impacts from material and process parameters- addressing cases such as the environmental trade-offs between molten salts and hydrofluoric acid, or the use of bio-sourced activated carbon electrodes.
Ultimately, this work contributes to building a new generation of AI-driven LCA tools that can accelerate the design of cleaner, more sustainable materials for the renewable energy transition.
Researcher: Javid Isayev
Seawater electrolysis with integrated brine valorisation
As global demand for freshwater continues to rise, seawater desalination has become a vital technology, particularly in Mediterranean countries. However, this process generates large volumes of brine — a concentrated salt solution often discharged back into the sea, harming marine ecosystems through salinity gradients and biodiversity loss.
Rather than viewing brine as waste, this research explores its potential as a valuable resource. The project investigates how the ions naturally present in brine can be used to produce hydrogen through electrolysis — a clean process powered by renewable energy such as solar power. This integrated approach links water desalination and green hydrogen production, contributing to the broader water-energy nexus.
By reimagining how brine is managed, this research aims to make desalination not only a solution for water scarcity but also a contributor to a more sustainable and resource-efficient future.
Researcher: Raphaël Defroyennes
Design of a Sustainable Proton Exchange Membrane for Green Hydrogen Production from Wastewater using an Integrated Microbial Fuel Cell-Microbial Electrolysis Cell (MFC-MEC) System
This research aims to develop a cost-effective, high-performance, and fluorine-free PEM as an alternative to Nafion for application in dual-chamber integrated MEC and MFC systems. The study will focus on exploring several alternatives, such as hydrocarbon-based and/or biosourced polymers, due to their excellent thermal stability, mechanical robustness, carbon footprint, and chemical resistance. To enhance proton conductivity and ion exchange capacity, modification of these polymers will be investigated to introduce proton-conducting functional groups, such as sulfonic acid (-SO3H). Additionally, nanomaterials such as graphene, carbon nanotubes (CNTs), and/or metal-organic frameworks (MOFs) will be incorporated into the membrane matrix to improve ionic conductivity, mechanical strength, and thermal stability, resulting in a mixed-matrix membrane (MMM).
Researcher: Andreas