We and others have shown that several endocannabinoids and related lipids play an important role in inflammation.
We have shown that increasing 2-AG levels via MAGL inhibition reduces colitis in a partially CB1- and CB2-dependent manner (Alhouayek et al. FASEB J., 2011). We also showed that inhibition of ABHD6 increases 2-AG levels in some tissues, and has pronounced anti-inflammatory effects in vivo (Alhouayek et al. PNAS, 2013).
We showed that the ABHD6 inhibitor WWL70 strongly decreases all the hallmarks of lung inflammation (including neutrophil infiltration, cytokine secretion, and protein extravasation) induced by intratracheal administration of LPS, a model of acute lung injury. As macrophages and neutrophils are key cells in acute lung inflammation, we also studied ABHD6 inhibition on primary alveolar macrophages and neutrophils to explore their potential implication in the effects observed in vivo (Bottemanne et al. FASEB J, 2019).
We also demonstrated in vitro that ABHD6 inhibition in activated macrophages favors the production of PGD2-G, a bioactive lipid derived from 2-AG (Figure 2), that we found to have potent anti-inflammatory effects (Alhouayek et al. PNAS, 2013).

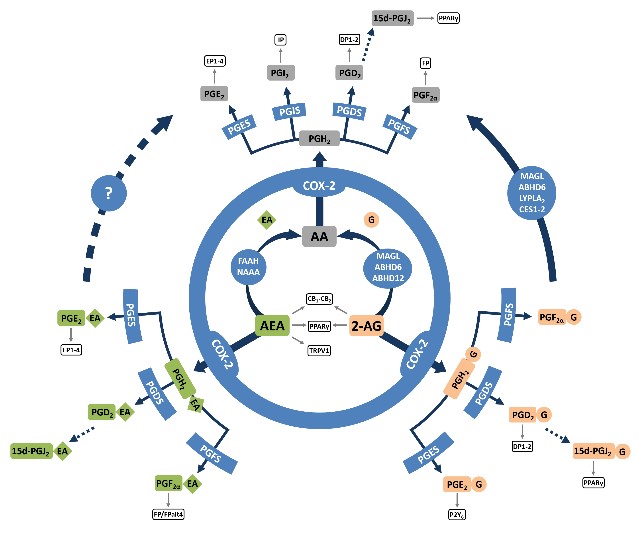
Demonstrating that PGD2-G has anti-inflammatory properties (Alhouayek et al. PNAS, 2013) opened several research projects in our research group. For instance, we demonstrated that administration of PGD2-G to mice having DSS-induced colitis allows for a strong reduction of the major hallmarks of the disease. Moreover, we could put forth the DP1 receptor as one of the receptors mediating the effects of PGD2-G in the colon (Alhouayek et al. FASEB J., 2018).
Additionally, in the context of a PhD thesis (supervisors Prof. des Rieux (ADDB) & Prof. Muccioli), we have developped an innovative formulation for PGD2-G, with the aim of increasing its delivery and efficacy in the CNS. Promising results obtained in a murine model of multiple sclerosis, the experimental autoimmune encephalomyelitis (EAE) model, support the feasibility and interest of this approach (Mwema A. et al. Nanomedicine. 2022).
Because inflammation can lead to painful sensations, we also asked whether PGD2-G could reduce inflammation-induced pain. We found that PGD2-G decreased hyperalgesia and edema in carrageenan-induced inflammatory pain, leading to a faster recovery. PGD2-G also decreased carrageenan-induced inflammatory markers in the paw as well as inflammatory cell recruitment. The effects of PGD2-G were independent from metabolite formation (PGD2 and 15d-PGJ2-G) or DP1 receptor activation in this model (Buisseret et al. BBA Mol. Cell Biol. Lipids, 2019). In a follow up study, we investigated the effects of the COX2-derived endocannabinoid metabolites (i.e. prostamides (PG-EAs) and prostaglandin glycerol esters (PG-Gs) on LPS-induced and carrageenan-induced hyperalgesia. Moreover, we compared in the same models the effect of the S- and R- enantiomers of flurbiprofen, the latter considered as a substrate selective COX inhibitor (Buisseret et al. FASEB J, 2021).
Besides these examples, our continuing efforts will contribute to highlight further the interest of modulating endocannabinoids and related lipids’ levels in inflammatory situations. For instance, we are actively collaborating with Dr Makriyannis and Dr Malamas (Northeastern University, Boston) on the characterization of the role of NAAA in inflammation (Alhouayek et al. FASEB J, 2015; Alhouayek et al. BBA Mol. Cell Biol. lipids, 2017).
Case in point, we assessed the effect of NAAA inhibition in the EAE mouse model of multiple sclerosis. Our results show that NAAA inhibition decreases inflammation and demyelination in this model (Figure 3). Interestingly, this was not the case with FAAH inhibition in the same setting. (Bottemanne et al. Neurotherapeutics 2021).





